ABSTRACT
The Csp2-O bond formation via direct oxidation of the Csp2-H bond on electron-rich compounds such as naphthols, is a process that generally requires drastic reaction conditions like high temperature or pressure. Addition of strong oxidants as H2O2, hypervalent iodine reagents (λ3 o λ5), expensive transition metals or rare earth elements, such as Mo, Ru Pt or Ce, is usually necessary. As part of this study on oxidative dimerization of phenols towards the total synthesis of ningalin D, 1,3-naphthalenediol was explored as starting material using stoichiometric amounts of Cu(I) and atmospheric molecular oxygen. A novel two-step sequence reaction for the formation of a 1,4-naphthoquinone was found instead of a dimerization product. The synthesis of this compound involves two consecutive oxidation processes.
RESUMEN
La formación del enlace Csp2-O vía oxidación directa del enlace Csp2-H de compuestos ricos en electrones como los naftoles, es un proceso que generalmente necesita condiciones drásticas de reacción como temperatura y presiones elevadas. Además oxidantes fuertes como H2O2, reactivos de yodo hipervalente (λ3 o λ5), metales de transición costoso o de las tierras raras como Mo, Ru, Pt o Ce suelen ser necesarios. Como parte de nuestro estudio en la dimerización oxidativa de fenoles hacia la síntesis total de la ningalina D, se exploraron 1,3-naftalendioles como materiales de partida. En este documento se describieron por primera vez una oxidación eficiente, escalable y económica del 3-fenil-1,3-nafatlendiol utilizando cantidades estequiométricas de Cu(I) y oxígeno molécular atmosférico. Encontramos una novedosa secuencia de dos oxidaciones consecutivas en un solo paso de reacción para la formación de una 1,4-naftoquinona. La síntesis de este compuesto involucra dos procesos consecutivos de oxidación.
INTRODUCCIÓN
Oxidation processes on organic molecules are important transformations. Several examples of organic oxidations can be pointed out (Minisci, Citterio, Vsaimara, Fontana & De Bernardinis, 1989); however, those implying the homo-coupling of a single molecular unit to generate symmetric dimers are of special relevance. In this sense, it is possible to highlight only few relevant C2-symmetric naturally occurring compounds (He et al., 2012) (figure 1).
|
|
|
 |
|
|
Figure 1. Relevance of the C2-symmetry highlighted by some naturally occurring organic structures.
Source: Author´s own elaboration.
|
 |
Figure 1. Relevance of the C2-symmetry highlighted by some naturally occurring organic structures.
Source: Author´s own elaboration.
Close |
As illustrated in figure 1, very important compounds are identified as organic dimers, which attract the synthetic chemistry industry attention for developing oxidative homo-dimerization procedures.
Among the great amount of oxidative homo-dimerization protocols (Dohi, Takenaga, Goto, Maruyama & Kita, 2007) described up to date (Li et al., 2010), the transition metal-catalyzed procedures have been continuously used in different publications. Among them, we can mention methodologies (Monguchi, Yamamura, Fujiwara, Somete & Mori, 2010) using Cu(I) (Sabbasani & Lee, 2015), Fe(III) (Smith, Nawrat & Moody, 2011), Mo (V) (Moritz & Siegfried, 2016) or Ce(IV) (Shukla, Rani & Tewari, 2012) as relevant examples (figure 2).
|
|
|
 |
|
|
Figure 2. Representative examples for oxidative homo-coupling of aryl using different metals.
Source: Author´s own elaboration.
|
 |
Figure 2. Representative examples for oxidative homo-coupling of aryl using different metals.
Source: Author´s own elaboration.
Close |
Copper-catalyzed enantioselective dimerization of the 2-acetoxy-3-hydroxynaphthalene derivative takes place under oxygen atmosphere conditions (figure 2a). Another assay in similar conditions was developed using Fe(III) as a catalyst and atmospheric oxygen (figure 2b); in this case, a 2-phenyl group is present in the starting material. Finally, an exquisite application of ammonium cerium(IV) nitrate in the naturally occurring total synthesis of hybocarpone is illustrated (figure 2c).
As revised, the target homodimers can successfully be synthesized. Specifically, in naphthalenes, an hydroxyl group adjacent to de reaction site was necessary.
On the other hand, for the ‘Discussion’ section in this manuscript, it is important to mention some procedures regarding the direct C-O bond formation in aryls. In this sense, we can cite the work of Rosen et al. (2013), as well as Gallardo-Donaire & Martin (2013) and Novák, Correa, Gallardo-Donaire & Martin (2011) as significant representatives (figure 3).
|
|
|
 |
|
|
Figure 3. Direct Csp2-O bond formation by palladium- and copper-catalyzed reactions
Source: Author´s own elaboration.
|
 |
Figure 3. Direct Csp2-O bond formation by palladium- and copper-catalyzed reactions
Source: Author´s own elaboration.
Close |
Palladium-catalyzed acetoxylation of (+)-methylchromasonarol derivative followed by Lewis acid mediated amide hydrolysis give rise to a hydroxylation product (figure 3a). Moreover, the copper-catalyzed 6H-benzo[c]chromen-6-one formation, from 2-arylbenzoic acids and concomitant hydrolysis, yield the corresponding hydroxylation (figure 3b). Finally, the direct acetoxylation of benzene derivatives, using triazoles as directing groups, lets to the direct C-O bond formation.
As part of this program towards the synthesis of molecules in cancer chemotherapy, the naturally occurring alkaloid ningalin D (Hamasaki, Zimpleman, Hwang & Boger, 2005) (figure 1) was identified as an excellent candidate in the treatment against multidrug resistance (MDR) cancer. The total synthesis of the alkaloid implies an oxidative aryl homo-dimerization as the key step, based upon the logical chemistry of its symmetrical architecture. The retrosynthetic analysis is outlined as follows:
This synthetic plan involves the synthesis of 1,3-naphthalenediol 4, with the properly functionalization, which would undergo oxidative homo-dimerization, yielding the key advanced intermediate 3. Finally, double 1,4-addition-elimination 2 and hydroxy group deprotection would give rise to the total synthesis of ningalin D, 1.
MATERIALS AND METHODS
Complete details on the synthesis of obtained compounds, as well as analytic and spectroscopic characterization data, are given in this section.
The glass material was dried with a heat gun previous to use, according to the Schlenk technique. The solvents (PhMe, MeCN, EtOH, MeOH) were anhydrous and deoxygenated.
Thin layer chromatography was carried out on silica gel supported on aluminum foil (0.25 mm). Adimensional retention factor (Rf) is reported. Purification of crude of reactions was carried out using column chromatography with a stationary phase in which silica gel (60-200 mesh size) is used as adsorbent.
The 1H and 13C NMR spectra were recorded in a pair of Bruker's AscendTM spectrometers (400 MHz and 500 MHz) using CDCl3. The chemical shifts (δ) for acquired spectra were reported relative to 0.0 ppm considering the TMS signal, or to 7.27 ppm by the residual signal in CDCl3.
Spectroscopic data are reported in the following order: chemical shift in ppm (δ), multiplicity, coupling constant in Hz (J) and integration. The multiplicities are reported as s (singlet), d (doublet), dd (doublet of doublets), t (triplet), c (quadruplet) or m (multiplet).
The melting points were determined using a Fisher-Jones apparatus and they are reported in Celsius degrees (°C).
RESULTS
In accordance with figure 4, the first step towards the synthesis of ningalin D is the preparation of the 1,3-naphthalenediol 4. A strongly recommended strategy, in total synthesis, is to develop a model system. The model systems are molecules structurally close related to the target, whose synthesis is easier and allows the evaluation of a promising or uncertain result in the complete route. Considering this idea, we decided to carry out the synthesis of a model molecule towards the preparation of ningalin (figure 4).
|
|
|
 |
|
|
Figure 4. This synthetic strategy for the synthesis of ningalin D, sticking out the oxidative homo-dimerization as key step.
Source: Author´s own elaboration.
|
 |
Figure 4. This synthetic strategy for the synthesis of ningalin D, sticking out the oxidative homo-dimerization as key step.
Source: Author´s own elaboration.
Close |
Thus, ningalin D analogue 5, would be prepared by double Michael addition-elimination of phenethylamine B in 6. Methylation of 7 will activate the free hydroxyl groups as better leaving groups to the aforementioned 1,4-addition-elimination reaction. As a consequence, 7 could be prepared via oxidative homo-dimerization of 8. Clearly it is possible to realize that 8 is a closely related to 4 (figure 5).
|
|
|
 |
|
|
Figure 5. Model system strategy towards this proposed total synthesis of ningalin D.
Source: Author´s own elaboration.
|
 |
Figure 5. Model system strategy towards this proposed total synthesis of ningalin D.
Source: Author´s own elaboration.
Close |
Thereby, everything started synthesizing 8 (figure 6).
|
|
|
 |
|
|
Figure 6. Synthesis of the naphthalenediol 8.
Source: Author´s own elaboration.
|
 |
Figure 6. Synthesis of the naphthalenediol 8.
Source: Author´s own elaboration.
Close |
The route begins with the synthesis of 9 by reacting commercial inexpensive benzyl chloride with potassium cyanide in EtOH-H2O solvent system, under refluxing conditions. Remarkably, the step occurs on a hundred-gram scale and 79% yield. Nitrile hydrolysis and concomitant esterification give rise to the compound 10 in 84%. Claisen reaction between two single units of 10 in presence of sodium hydride produces 11 which is not purified and directly reacted with concentrated sulfuric acid yielding 8 in 42% yield. This reaction takes place on at least a five-gram scale allowing the preparation of several batches. It is worthy to highlight that this first route to 8 was carried out in multigram scale. This is an important feature to embark on a total synthesis program.
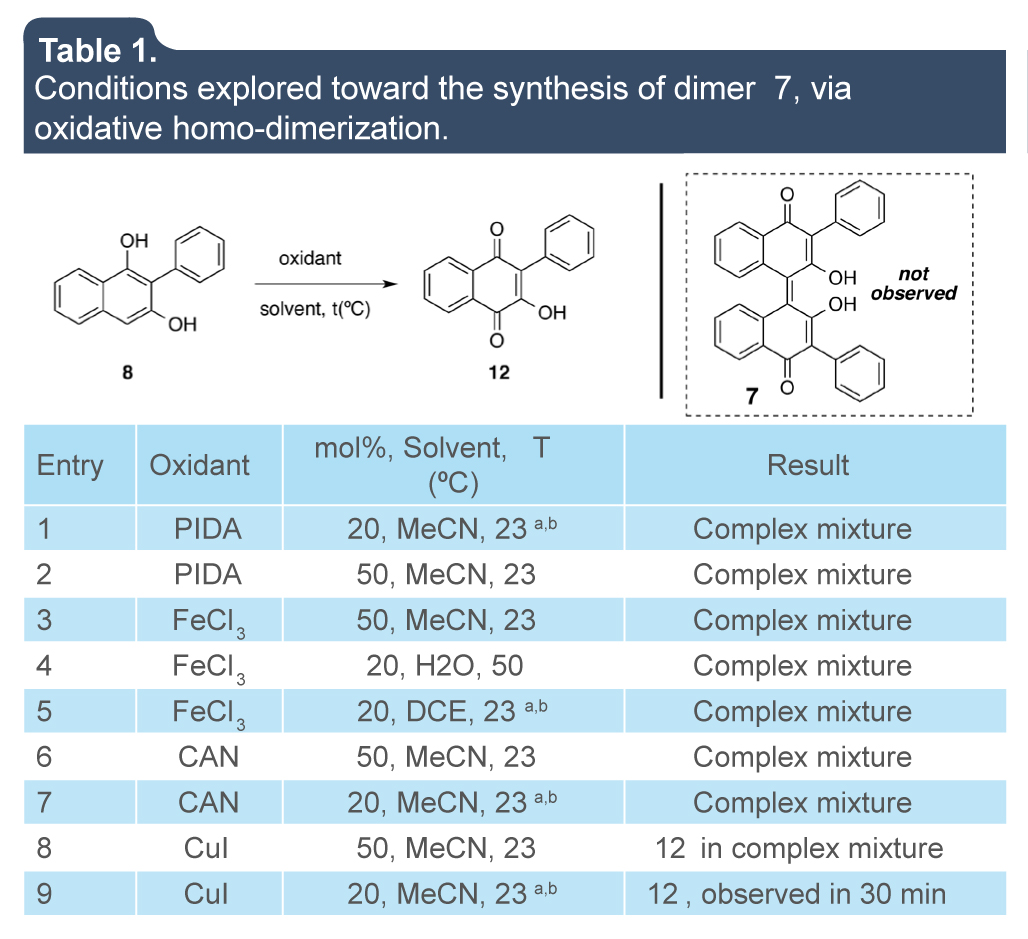
Once naphthol 8 synthesized, the oxidative homo-dimerization was the next step according to this strategy (figure 5). It was decided to explore transition metals as well as known organic oxidants (table 1).
Table 1.
Conditions explored toward the synthesis of dimer 7, via oxidative homo-dimerization.
|
|

|
|
Entry
|
Oxidant
|
mol%, Solvent,
T (ºC)
|
Result
|
|
1
|
PIDA
|
20, MeCN, 23a,b
|
Complex mixture
|
|
2
|
PIDA
|
50, MeCN, 23
|
Complex mixture
|
|
3
|
FeCl3
|
50, MeCN, 23
|
Complex mixture
|
|
4
|
FeCl3
|
20, H2O, 50
|
Complex mixture
|
|
5
|
FeCl3
|
20, DCE, 23a,b
|
Complex mixture
|
|
6
|
CAN
|
50, MeCN, 23
|
Complex mixture
|
|
7
|
CAN
|
20, MeCN, 23a,b
|
Complex mixture
|
|
8
|
CuI
|
50, MeCN, 23
|
12 in complex mixture
|
|
9
|
CuI
|
20, MeCN, 23a,b
|
12, observed in 30 min
|
a Oxygen atmosphere was used. bThe progress of the reaction was followed by TLC along four days.
Source: Author´s own elaboration
Abrir
 |
a Oxygen atmosphere was used. bThe progress of the reaction was followed by TLC along four days.
Source: Author´s own elaboration Close |
When the homo-dimerization reaction assays were carried out with PIDA (entries 1-2), only complex mixtures were observed. The use of FeCl3 (entries 3-5) or CAN (entries 6-7) only gave rise to decomposition of the starting material or complex mixture reactions. The use of CuI in either 20 mol% or 50 mol% clearly allowed the observation of a new formed spot. After a complete and careful characterization, it was determined that compound 12 is formed instead of 7. The best conditions were found to be 20 mol% of CuI in presence of oxygen atmosphere.
To fully confirm the synthesis of compound 12, at least two large scale preparation on 1 g and 2 g were accomplished by using the previously developed conditions (equation 1).
In that way, and also by comparison, the scalable preparation of compound 12 was confirmed, since the quinone in question had been already synthesized.
DISCUSSION
The previously described results represent a totally different transformation to the desired one. The synthesis of this 2-hydroxy-3-phenyl 1,4-naphthoquinone implies the direct formation of a new Csp2-O bond and subsequent oxidation to generate the 1,4-naphthoquinoid system. To the best of this knowledge, this is the first example of a scalable oxidation on electron-rich naphthalenes using only Cu(I) and O2 as oxidants, and generating 1,4-naphthoquinones. This novel transformation has no precedent in copper-catalyzed direct C-O bond formation reactions. Even though similar protocols have been reported, in those cases cobalt-complexes with salen ligands were described as catalysts.
While an experimental evidence for an unequivocal mechanism requires additional studies, here in it is proposed a plausible reaction pathway, based upon the known chemistry of copper (Didziulis, Butcher, Cohen & Solomon, 1989) (figure 7).
|
|
|
 |
|
|
Figure 7. Proposed reaction pathway in the Cu(I)/O2-mediated oxidation of 1,3-naphthalendiol to 2-hydroxy-1,4-naphthoquinone.
Source: Author´s own elaboration.
|
 |
Figure 7. Representative examples for oxidative homo-coupling of aryl using different metals.
Source: Author´s own elaboration.
Close |
The mechanism proposes the CuI to CuII oxidation by atmospheric oxygen (Didziulis et al., 1989). Thus copper (II) generates radical I/I-A which traps molecular oxygen giving rise to the radical specie II. Then reduction of CuII to CuI produces the copper(I) peroxide III. The following tautomerization produces IV in [1,5] metallotropic equilibrium with V. Finally the peroxide V, promote another redox cycle CuI to CuII which oxidize the hydroquinone to observed 1,4-naphthoquinone releasing CuI that get into another catalytic cycle.
Even though a [4+2] cycloaddition between 8 and O2 can be plausible, at this point we ruled it out on account of two reasons: 1) This is not a reaction with an atmosphere rich in O2, but molecular oxygen comes only from air which is a mixture of some other gases. 2) Naphthoquinones are not totally good dienes in a [4+2] cycloaddition, which imply that for achieving success in such a reaction under this pathway, additionally to Cu used as a catalyst, an abundant source of molecular oxygen would be necessary; nevertheless, this oxygen source is not present.
CONCLUSIONS
In conclusion, here in it was described the first example in a novel oxidation of a 1,3-naphtalenediol using only Cu(I) and molecular O2 from air. The procedure was successfully applied to the gram scale synthesis of the 2-hydroxy-3-phenyl-1,4-naphthoquinone. In regards to synthetic chemistry, the developed reaction features low cost, easy manipulation, efficiency and no high atmospheric pressures. Studies on the future scope are currently on going in this laboratory.
ACKNOWLEDGMENTS
We gratefully thank FOMIX Consejo Nacional de Ciencia y Tecnología-Consejo de Ciencia y Tecnología del Estado de Guanajuato (Conacyt-Concyteg) GTO-2012-C03-194610 for financial support. We acknowledge the facilities from the DCNyE Chemistry Department at University of Guanajuato in the National Laboratory UG-CONACyT (LACAPFEC) for full characterization. We thank CONACyT for granting some fellowships to I. J Arroyo-Córdoba.
REFERENCES
Didziulis, S. V., Butcher, K. D., Cohen, S. L., & Solomon, E. I. (1989). Chemistry of Copper Overlayers on Zinc Oxide Single-Crystal Surfaces: Model Active Sites for Cu/ZnO Methanol Synthesis Catalysts. Journal of the American Chemical Society, 111, 7110-7123.
Dohi, T., Takenaga, N., Goto, A., Maruyama, A., & Kita, Y. (2007). Direct Lactone Formation by Using Hypervalent Iodine(III) Reagents with KBr via Selective C−H Abstraction Protocol. Organic Letters, 9(16), 3129–3132.
Gallardo-Donaire, J., & Martin, R. (2013). Cu-Catalyzed Mild C(sp2)–H Functionalization Assisted by Carboxylic Acids en Route to Hydroxylated Arenes. Journal of the American Chemical Society, 135(25), 9350-9353.
He, H. Y., Pan, H. X., Wu, L. F., Zhang, B. B., Chai, H. B., Liu, W., & Tang, G. L. (2012). Quartromicin Biosynthesis: Two Alternative Polyketide Chains Produced by One Polyketide Synthase Assembly Line. Chemistry & Biology, 19, 1313-1323..
Hamasaki, A., Zimpleman, J. M., Hwang, I., & Boger, D. L. (2005). Total Synthesis of Ningalin D. Journal of the American Chemical Society, 127(30), 10767-10770.
Li, Y., Wang, W. H., Yang, S. D., Li, B. J., Feng, C., & Shi, Z. J. (2010). Oxidative dimerization of N-protected and free indole derivatives toward 3,3-biindoles via Pd-catalyzed direct C–H transformations. Chemical Communications, 46, 4553-4555.
Minisci, F., Citterio, A., Vsaimara, E., Fontana, F., & De Bernardinis, S. (1989). Facile and convenient syntheses of quinones from phenols. The Journal of Organic Chemistry, 54(3), 728-732.
Monguchi, D., Yamamura, A., Fujiwara, T., Somete, T., & Mori, A. (2010). Oxidative dimerization of azoles via copper(II)/silver(I)-catalyzed CH homocoupling. Tetrahedron Letters, 51, 850-852.
Moritz, S., & Siegfried, R. W. (2016). MoV Reagents in Organic Synthesis. European Journal of Organic Chemistry, 1921-1936.
Novák, P., Correa, A., Gallardo-Donaire, J., & Martin, R. (2011). Synergistic Palladium-Catalyzed C(sp3)-H Activation/C(sp3)-O Bond Formation: A Direct, Step-Economical Route to Benzolactones. Angewandte Chemie International Edition, 50, 12236-12239.
Rosen, B. R., Simke, L. R., Thuy-Boun, P. S., Dixon, D. D., Yu, J. Q., & Baran, P. S. (2013). C-H Functionalization Logic Enables Synthesis of (+)-Hongoquercin A and Related Compounds. Angewandte Chemie International Edition, 52, 7317-7320.
Sabbasani, V. R., & Lee, D. (2015). Oxidative Dimerization of Silylallenes via Activation of the Allenic C(sp2)–H Bond Catalyzed by Copper(I) Chloride and N-Hydroxyphthalimide. Organic Letters, 17, 4878-4881.
Shukla, R., Rani, S., & Tewari I. C. (2012). Synthesis and characterization of some new diorganobismuth (III) aryloxyacetates and their antimicrobial screening. International Journal Chemistry Research, 24, 122-125.
Smith, M. J., Nawrat, C. C., & Moody, C. J. (2011). Synthesis of Parvistemin A via Biomimetic Oxidative Dimerization. Organic Letters, 13, 3396-3398.
ANEXO
Procedure of synthesis
In a reaction two-neck flask equipped with a condenser and a septum, the chlorobenzyl (100 g, 0.7821 mol, 1 equiv.) was diluted in 450 mL of ethanol. A solution of KCN (160 g, 2.346 mol, 3 equiv.) in 150 mL of water is added. The mixture is over-night refluxed. After that, the ethanol was reduced under vacuum. The organic phase of the crude is separated. The organic phase was washed with water (3 mL × 150 mL) and the original aqueous phase was washed with diethyl ether (3 mL × 150 mL). The combined organic phases were dried with Na2SO4 and the resulting solution is evaporated under vacuum. The resulting compound was purified by flash column chromatography (SiO2, 20% EtOAc in hexanes). The fraction obtained is evaporated under vacuum to yield the product as a light yellow oil (79%). Rf = 0.49 (20% EtOAc/hexanes). 1H NMR (CDCl3, 400 MHz): δ 7.37 (m, 5 H), 3.72 (s, 2 H). 13C NMR (100 MHz, CDCl3): 130.04, 128.95, 127.80, 23.25.
In a reaction two-neck flask equipped with a condenser and a septum, the 2-phenylacetonitrile 9 (70 g, 0.5975 mol, 1 equiv.) was diluted in 150 mL of ethanol and 50 mL of concentrated sulfuric acid was carefully added. The mixture is over-night refluxed. After that, the ethanol was complete reduced under vacuum. The resulting mixture is carefully poured on an ice bath, the mixture was allowed to reach room temperature. The organic phase of the crude is separated. The organic phase was washed with water (3 mL × 150 mL) and the original aqueous phase was washed with diethyl ether (3 mL × 150 mL). The combined organic phases were dried with Na2SO4 and the resulting solution is evaporated under vacuum. The resulting compound was purified by flash column chromatography (SiO2, 20% EtOAc in hexanes). The fraction obtained is evaporated under vacuum to yield the product as a yellow liquid (84%). Rf = 0.67 (20% EtOAc/hexanes). 1H NMR (CDCl3, 400 MHz) δ 7.32 (m, 5 H), 4.19 (q, J = 4.0 Hz, 2 H), 3.66 (s, 2 H), 1.29 (t, J = 8.0 Hz, 3 H). 13C NMR (100 MHz, CDCl3): 171.44, 134.17, 129.19, 128.48, 126.96, 60.70, 41.32, 14.30.
In a dry reaction two-neck flask equipped with 2 septum, sodium hydride (2.7 g, 0.0670 mol, 1.1 equiv.) was diluted with 25 mL of dry THF under nitrogen atmosphere at room temperature. The ethyl 2-phenylacetate 10 (10 g, 0.0609 mol, 1 equiv.). was dropwise added. The reaction was warmed at 65 °C and stirred by 2 h. After that, the reaction was allowed to reach room temperature and then was cooled in an ice bath. At 0 °C, the reaction mixture was quenched with a solution of 15 mL of concentrated hydrochloric acid in 10 mL of water. The crude was extracted with EtOAc (3 mL × 50 mL). The organic phase was dried with Na2SO4 and the resulting solution is evaporated under vacuum to yield the product as a dark oil (~54%) that was used without further purification. 1H NMR (CDCl3, 500 MHz) δ 7.45 (m, 4 H), 7.34 (m, 4 H), 7.10 (m, 2 H), 4.83 (s, 1 H), 4.16 (q, J = 7.1 Hz, 2 H), 3.68 (s, 2 H), 1.29 (t, J = 7.1 Hz, 3 H).
In a reaction flask the ethyl 3-oxo-2,4-diphenylbutanoate 11 (10 g, 0.0121 mol, 1 equiv.) and 30 mL of concentrated sulfuric acid were added. The mixture was stirred over-night at room temperature. The reaction was quenched with 200 mL of ice-water, the mixture was allowed to reach room temperature. The solid was filtered and the aqueous layer was extracted with EtOAc (3 mL × 250 mL). The solid was diluted with EtOAc and washed with water (3 mL × 100 mL). The combined organic phases were dried with Na2SO4 and the resulting solution is evaporated under vacuum. The resulting compound was purified by flash column chromatography (SiO2, 5% EtOAc in hexanes). The fraction obtained is evaporated under vacuum to yield the product as a brown solid (42%). 1H NMR (CDCl3, 500 MHz) δ 8.06 (d, J = 8.4 Hz, 1 H), 7.59 (d, J = 8.3 Hz, 1 H), 7.53 (dd, J = 10.4, 4.6 Hz, 2 H), 7.45 (dd, J = 7.9, 1.6 Hz, 1 H), 7.41 (dd, J = 5.1, 3.1 Hz, 2 H), 7.37 (m, 1 H), 7.25 (ddd, J = 8.1, 6.9, 1.1 Hz, 1 H), 6.87 (s, 1 H). 13C NMR (CDCl3, 125 MHz) δ 151.19, 149.38, 134.82, 131.36, 131.19, 130.61, 129.63, 127.46, 126.37, 123.26, 122.69, 113.45, 102.42.
In a reaction vessel was diluted the naphthol 8 (2 g, 0.0085 mol, 1 equiv.) with 10 mL of dry MeCN. The CuI (329 mg, 0.0017 mol, 0.2 equiv.) was added in one portion. The mixture was stirred at room temperature by 30 minutes. The solvent was evaporated under reduced pressure. The resulting compound was purified by flash column chromatography (SiO2, 20% EtOAc in hexanes). The fraction obtained is evaporated under vacuum to yield the product as an orange needles (62%). 1H NMR (CDCl3, 500 MHz) δ 8.11 (d, J = 7.6 Hz, 2 H), 8.06 (d, J = 7.6 Hz, 2 H), 7.71 (t, J = 7.6 Hz, 1 H), 7.64 (m, 2 H), 7.56 (s broad, 2 H), 7.43 (m, 4 H), 7.37 (t, J = 7.5 Hz, 4 H), 7.31 (dd, J = 8.4, 6.2 Hz 2 H). 13C NMR (CDCl3, 125 MHz) δ 183.89, 182.05, 152.44, 135.46, 133.31, 133.05, 130.85, 130.18, 129.52, 128.84, 128.11, 127.47, 126.31, 122.37.